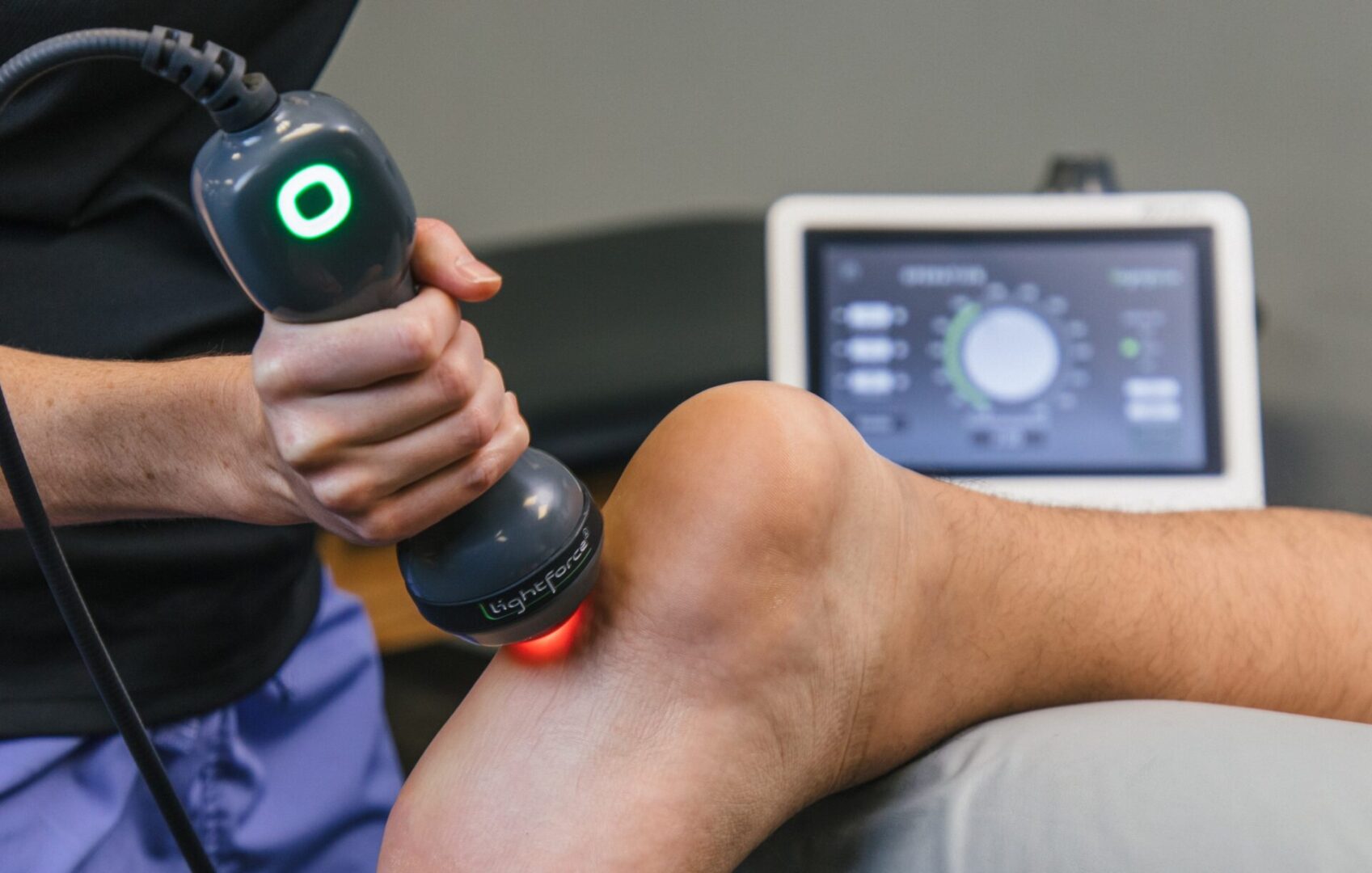
Revised 2023 APTA Clinical Practice Guidelines for Heel Pain – Plantar Fasciitis Upgrades Therapeutic Laser Recommendation
Clinicians should use laser therapy to decrease pain for acute and chronic plantar fasciitis Laser therapy received a grade B…

Clinicians should use laser therapy to decrease pain for acute and chronic plantar fasciitis Laser therapy received a grade B…

Contributed by Mark Callanen, PT, DPT, OCS Cash-based services should add unique and discernible value to your patient’s plan of…

DJO® Acquires LiteCure Laser Therapy Acquisition establishes Chattanooga® as the leader in high-power laser therapy Dallas, TX (December 11, 2020)…
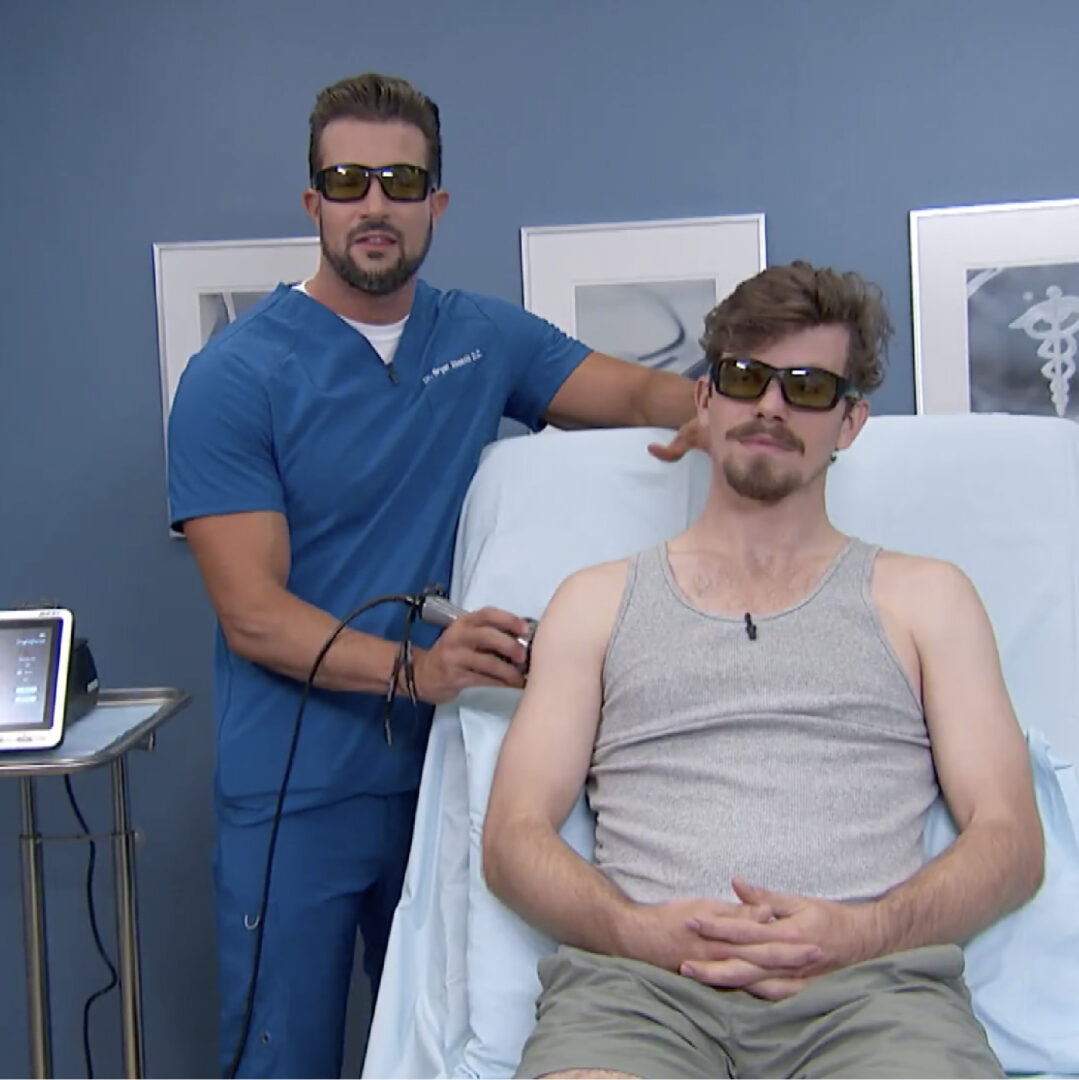
Learn what The Doctors have to say about laser therapy for pain. Watch Dr. Bryan Abasolo on the TV show,…

Contributed by Mark Callanen, PT, DPT, OCS Director of Clinical Development, LightForce® Therapy Lasers Inflammation is a topic that is…

What are the applications for different laser treatment heads? The following Exclusive LightForce Treatment Heads are available on all LightForce…
Contributed by Mark Callanen, PT, DPT, OCS Director of Clinical Development, LightForce® Therapy Lasers Being an orthopedic physical therapist for…
Check out our NEW WEBSITE We’ve redesigned our site with OUR CUSTOMERS IN MIND. Now find the latest…

Explaining how therapeutic laser can accelerate a plan of care. Contributed by Mark Callanen, PT, DPT, OCS Director of Clinical…

Contributed by Adam Marmon, Ph.D. Dangers of the Kitchen Knife There are a variety of tools that we use in…