Physical Exam
 Performing a systematic physical examination and visual observation of the patient’s mobility will provide an initial assessment on where the potential issue(s) may exist. A thorough orthopedic exam allows for palpation of the limbs and joints while moving them through their range of motion to draw attention to changes within the tissue and help assess if the patient seems painful. Additionally, it is important to complete a neurologic evaluation and consider running additional diagnostic tests to look for any underlying disease processes that could affect treatment options.
Performing a systematic physical examination and visual observation of the patient’s mobility will provide an initial assessment on where the potential issue(s) may exist. A thorough orthopedic exam allows for palpation of the limbs and joints while moving them through their range of motion to draw attention to changes within the tissue and help assess if the patient seems painful. Additionally, it is important to complete a neurologic evaluation and consider running additional diagnostic tests to look for any underlying disease processes that could affect treatment options.
Pain Evaluation
According to the Summary of 2015 AAHA/AAFP Pain Management Guidelines for Dogs and Cats, the most accurate method for evaluating pain is through observing any changes in the pet’s behavior. A pain score is considered the fourth vital sign after temperature, pulse and respiration.1 A patient’s pain should be evaluated in the clinic and by the pet owner to paint a full picture of how the pet is acting.
Pet Owner
Feedback from the owner is vital in understanding where, the length of time and severity of how the pet has been affected to determine the next steps for evaluating if arthritis is present. He or she should be asked a series of questions about any changes in behavior and given a pain scale form to fill out based on whether it is an acute or chronic problem. This will provide valuable insight into the pet’s everyday activities and how they vary.
Standing Evaluation
 Gathering data on how a patient is bearing weight while standing can provide objective numbers to determine which limb or limbs are effected. It has been shown that bathroom scales can be used as a reliable way to measure static weight bearing in canines2; therefore, a free-standing platform with built-in sensors, such as the Stance Analyzer, can visually show where a potential lameness exists. This sort of tool takes up minimal floor space and can be a cost-effective option in helping obtain a diagnosis, as well as measuring patient outcomes. Recording this sort of information can help guide treatment plans and provide owners with a better understanding of the source of their pets’ complications.
Gathering data on how a patient is bearing weight while standing can provide objective numbers to determine which limb or limbs are effected. It has been shown that bathroom scales can be used as a reliable way to measure static weight bearing in canines2; therefore, a free-standing platform with built-in sensors, such as the Stance Analyzer, can visually show where a potential lameness exists. This sort of tool takes up minimal floor space and can be a cost-effective option in helping obtain a diagnosis, as well as measuring patient outcomes. Recording this sort of information can help guide treatment plans and provide owners with a better understanding of the source of their pets’ complications.
Gait Analysis
Objectively measuring a patient’s gait can be performed via force plate or a commercially available portable walkway. Force plate is considered the gold standard for evaluating gait by calculating the ground reactive forces of a cat or dog while standing, walking or jogging over the plate. The portable walkway is a mat that allows a pet to stand, walk or jog providing information on gait symmetry, stride length and the amount of pressure placed on each paw. These types of devices are expensive and take up a lot of floor space, thus they are not as common of an option for general practice.
Imaging
It is very important to obtain a picture of the joints suspected of having osteoarthritis. Radiographs are most commonly taken to assess the bony changes associated with OA. MRI and CT offer additional diagnostic capabilities, though less commonly used to assess OA, with MRI showing how the soft tissues are involved, while CT can offer better detail for the bony changes in more complex joints.
Arthrocentesis
Collecting a sample of the fluid in a swollen joint or joints suspected of having arthritis can help rule out what is causing the swelling or pain, especially in younger patients, or in those with a more complicated clinical history. The color, consistency and cellular make-up of the synovial fluid can provide clues as to what changes are occurring within the joint and why.3
References
1. Epstein, M. et. al. (2015). 2015 AAHA/AAFP Pain Management Guidelines for Dogs and Cats. JAAHA, 51(2), 67-84. doi: http://dx.doi.org/10.5326/JAAHA-MS-7331. 2. Hyytiäinen, H.K. et. al. (2012). Use of bathroom scales in measuring asymmetry of hindlimb static weight bearing in dogs with osteoarthritis. Veterinary and Comparative Orthopaedics and Traumatology, 25(5), 390-396. doi: https://doi.org/10.3415/VCOT-11-09-0135. 3. Degner, D. (2014, August). Arthrocentesis in Dogs. Clinician’s Brief. Retrieved from https://www.cliniciansbrief.com/article/arthrocentesis-dogs.
Welcome back to the Companion Animal Health Regenerative Medicine blog series! In case you missed Part 1, you can read it here. In today’s blog, we are going to answer two more of the most frequently asked questions surrounding Platelet Rich Plasma.
Question 1: What is a joint flare? How often are they seen post injection?
A joint flare is an inflammatory response in the joint due to a change in the synovial environment. Also referred to as Reactive Synovitis, joint flares can be seen after the injection or withdrawal of fluid into or from the joint. The most common characteristics are: swelling of the injected joint(s), heat emittance from the area and mild to moderate lameness. Typically lasting between 24-28 hours post injection, joint flares can be managed utilizing pain medication (excluding NSAIDs).
Joint flares are reported to occur between 15-20% of patients, but have not been reported to have a negative effect on the treatment outcome. If symptoms are lasting more than 72 hours, synovial fluid analysis is recommended in case there is an unlikely event of an immune moderated disease or septic joint.
Question 2: How long should I wait to laser the area after injecting Platelet Rich Plasma?
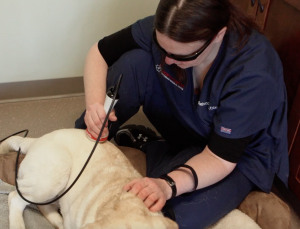 Currently there is limited data surrounding the interaction of photobiomodulation and PRP. However, preliminary research is beginning to show promise for the immediate use of laser post-injection. In one recent study, researchers investigated the use of photobiomodulation, platelet rich plasma and the combination of both in the healing of an Achilles tendon. The results of this study showed compounding therapeutic effects, shortening the healing time and returning the tissue closer to “normal” tissue structure.
Currently there is limited data surrounding the interaction of photobiomodulation and PRP. However, preliminary research is beginning to show promise for the immediate use of laser post-injection. In one recent study, researchers investigated the use of photobiomodulation, platelet rich plasma and the combination of both in the healing of an Achilles tendon. The results of this study showed compounding therapeutic effects, shortening the healing time and returning the tissue closer to “normal” tissue structure.
While this study shows promise, further research and validation in canine patients will provide more accurate recommendations for post injection settings and protocols. Currently it is recommended to wait 3-4 days post injection to begin treating the area with photobiomodulation.
If you would like to learn more about Platelet Rich Plasma and Stem Cell therapies or if you have questions that were not answered in this blog, please contact Companion Animal Health at info@companiontherapies.com.
Stay tuned for our next blog which will explore the benefits of treating with Platelet Rich Plasma!
Welcome back to the Companion Animal Health Regenerative Medicine blog series! In today’s blog, we are going to answer three of the most frequently asked questions regarding Platelet Rich Plasma. From protocols to assessing treatment response, we will provide clarity to some of the questions surrounding this ground-breaking therapy.
Question 1: Is there any proof that Platelet Rich Plasma therapy works?
This is a very common question as there are numerous sources of information regarding the efficacy of PRP treatments. A google search will provide different answers to PRP treatments and applications, though many may not be scientifically backed or peer-reviewed (the gold standard of validating a therapy). The number one place to look for efficacy data is the pubmed.gov website. Performing a search on this website will provide the latest peer-reviewed publications which investigate efficacy (or failure) of different treatments. We have provided several of these published papers for your review, which discuss the successful treatments utilizing Platelet Rich Plasma in numerous indications and may be accessed here.
Question 2: How soon after injecting Platelet Rich Plasma do you typically see results?
It is generally accepted both in human and veterinary medicine that positive effects of this therapy will become apparent within 5-10 days post treatment.
Question 3: How do I know if the patient is responding to treatment?
There are several considerations that need to be made when trying to decipher if a patient is responding to treatment. It is important to have a method for measuring a baseline of the patient and their response to a treatment. Many practitioners using Platelet Rich Plasma therapy will have patients return to the hospital 2 weeks post-treatment and will utilize on of the following methods to measure response.
- Canine Brief Pain Survey for Pet Owners – Created by the University of Pennsylvania, this survey asks the pet owner to grade their dog’s pain and general habits which may indicate improvement from the treatment. To access this survey (for free), click here.
- Pain Assessment/Palpation – Palpation is an extremely valuable tool when assessing a dog’s pain and locating the area of injury. Palpation can not only serve as a useful diagnostic, but can also provide feedback post treatment as to if the pain is still present or has reduced from its original state.

- Stance or Gait Analysis – Having a quantifiable measurement both pre-and post-treatment will provide you the best data for if a dog is improving from a therapy. Stance and Gait analysis provide objective measurements of the dog’s percent weight bearing on each of its limbs, center of gravity and stride length (gait analysis only).
- The Stance Analyzer is a cost-effective, compact system which can easily be integrated into a hospitals diagnostic program. Requiring a small foot print for floor space (approximately 2’ by 3’), the Stance Analyzer provides accurate readings of percent weight bearing, center of gravity and weight with a minimal time commitment. Once a reading is obtained, results are easy to interpret and can be saved for each patient to track responses to therapies.
- A gait analysis system typically consists of a 20-foot walkway mat which provides a detailed analysis of stride, locomotion and other advanced objective readouts. This too is a great system for quantifiable readouts, but requires additional space to perform correctly. These systems are more expensive than the Stance Analyzer and are typically utilized by universities performing research and specialty hospitals. Click here to read more about gait analysis systems.
- Gait videos – If you don’t have a stance analyzer or gait analysis but would like a visual comparison for pre- and post-treatment effects, you can use video to document your patient’s success! With the advancement of technology for smart phones, there are now applications and settings on the camera to take slow motion videos. These videos can enable you to point out changes in gait, stride length and head bobbing. They can also provide you with before and after videos to then market your regenerative treatments to your clients.
Stay tuned for our next blog where we will answer additional questions focused on Platelet Rich Plasma. If you have a question you would like answered, please contact Companion Animal Health at info@companiontherapies.com.





